Chapter 15 A PRIMER ON PFAS
| Jurisdiction | United States |
[Page 15-1]
A PRIMER ON PFAS
JEFF KRAY is an environmental litigator at Marten Law LLP in Seattle, Washington. Over a 28 year career, Jeff has conducted numerous trials, litigated administrative hearings, and appeared in federal and state appeals. He is consistently ranked by his peers as a top environmental lawyer and has been recognized as a leader in the environmental field by inclusion in Chambers, Best Lawyers, International Who's Who of Environmental Lawyers, and other publications. Before joining Marten Law in 2003, Jeff spent over a decade in the Washington Attorney General's Office. Jeff's practice focuses on the neighborhood in which PFAS has become an urgent environmental issue; the intersection between water quality, water resources, and complex environmental litigation, including Clean Water Act and Safe Drinking Water Act permitting and regulatory compliance, and CERCLA (Superfund) site remediation. He is a frequent speaker and writer on PFAS. Jeff has represented public and private clients throughout the country, including municipal water suppliers and municipal waste operators impacted by PFAS contaminants.
Polyfluorinated and perfluorinated substances, more commonly known as PFAS, continue to garner attention in the media as a health and environmental risk to millions of people around the world.1 PFAS are found in myriad useful products, from clothing to food packaging to building materials, and have been found in drinking water supplies across the country at levels exceeding EPA health advisory levels. Investigating and regulating PFAS have become priorities for federal and state policy makers. PFAS in the environment, including in water supplies, have triggered numerous lawsuits. This paper provides a primer on PFAS' history and chemistry; the response by various stakeholders, including EPA, states, water suppliers, and the courts to PFAS contamination; and the likely impacts that PFAS regulation will have on site remediation and environmental litigation for years to come.
I. Background
PFAS is a generic term for a large family of synthetic, highly mobile, and persistent chemicals that do not break down in the environment. There is no universally accepted definition, but all PFAS contain a carbon-fluorine bond. They are broken down into polymer and non-polymer classes, all containing either a fully (per) or partly (poly) fluorinated carbon chain. PFAS can be further distinguished as short- or long-chain, based on the length of the that chain. PFAS have many valuable properties, including fire resistance and suppression, and oil, stain, grease, and water repellency.2 The chemicals were first developed in the 1930s, and, within 30 years could, be found in firefighting foams, wire insulation, cleaners, textiles, apparel, carpet, leather, paper, and paints.3 The first PFAS developed were long-chain, containing eight or more carbon atoms.4 These include the two most widely known PFAS: perfluorooctanoic acid (PFOA) and perfluorooctane sulfate (PFOS). Beginning in the 2000s, companies began to
[Page 15-2]
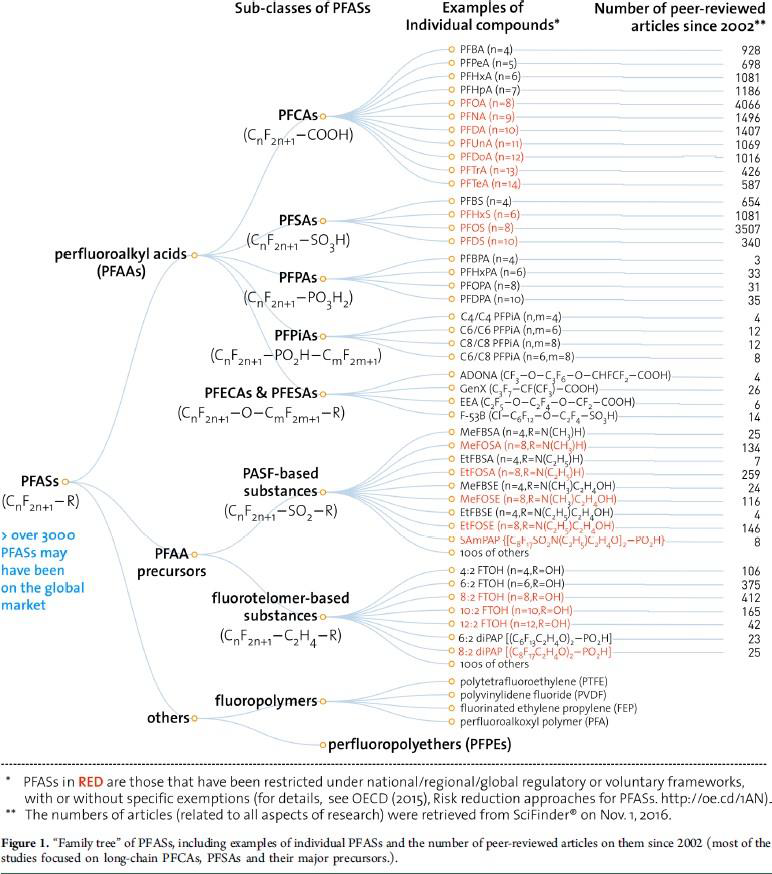
develop short-chain PFAS, meaning they have fewer than eight carbon atoms.5 GenX is one of the most well-known short-chain PFAS. As of May 2018, the Organisation for Economic Co-Operation and Development had identified 4,730 PFAS that may have been used on the global market since the late 1940s.6 By September 2020, the U.S. Environmental Protection Agency's (EPA) Master List of PFAS Substances contained 9,252 chemicals.7 Figure 1 below depicts PFAS subclasses and individual constituent examples per subclass. It also estimates the peer reviewed articles written on each PFAS example listed, indicating scientists have done more research on long chain PFAS.
Figure 1. "Family tree" of PFAS chemicals8
[Page 15-3]
Because of their widespread use and persistence (PFAS do not appear to degrade naturally),9 they are now found worldwide in the environment, wildlife, and humans.10 The serum of nearly every person in the United States that the Centers for Disease Control and Prevention (CDC) has tested since 1999 has contained PFOS, PFOA, PFHxS, and PFNA.11 According to industry human biomonitoring data, PFOA is also found in the blood of the general population in all geographic regions of the United States.12
A. Environmental and Health Risks Remain Under Study
The widespread presence and persistence of PFAS has the potential to be harmful to the environment and human health.13 With exposure, PFAS accumulate in the blood and liver. Because PFAS are not metabolized, they can bioaccumulate in terrestrial food webs and in marine mammals; thus, organisms higher in the food chain generally have higher PFAS levels than those lower in the food chain.14
Several studies have shown that PFAS, specifically the comparatively well-studied PFOA and PFOS, are associated with adverse health effects.15 Peer-reviewed studies on laboratory animals and epidemiological studies of human populations indicate that exposure to PFOA and PFOS over certain levels may result in developmental effects to fetuses and infants, cancer, and impacts to the liver, thyroid, immune system, and cholesterol changes.16 However, despite two decades of studies, "toxicologists are still struggling to work out exactly how PFAS cause problems in the body."17 This is only complicated by science continuing to identify new PFAS structures, each of which may cause different harms or work in a different way.18
B. PFAS Contamination Sources
To date, the two most well-characterized PFAS contamination sources are discharges from manufacturing plant and releases of aqueous film-forming foam (AFFF), designed and intended for use on fuel fires.
Many manufacturing facilities used PFAS starting in the 1950s. For example, in Parkersburg, West Virginia, DuPont used PFOA to make Teflon for over 40 years, resulting in PFOA powder releases into the Ohio River and sludge-containing PFOA releases into digestion ponds near the facility. PFOA entered the local water table, contaminating drinking water for more than 100,000 people.19 Similarly, in Hoosick Falls, New York, a manufacturing plant used PFOA to make stain resistant fabric. In a personal injury suit, the plaintiff alleges that employees discharged PFOA by dumping trays of cleaning residue containing PFOA into
[Page 15-4]
drains, which contaminated soil, groundwater, and ultimately the town's public water supply.20 Similar drinking water contamination originating from manufacturing plants has been discovered across the country, including in Minnesota, Alabama, Vermont, New Hampshire, and New Jersey.
Drinking water on and around military installations and civilian airports has been contaminated with PFAS due largely to the use of AFFF for training and fighting fuel fires. Although Department of Defense (DOD) memoranda indicate that DOD knew about the possible risks of PFAS in AFFF since the early 1980s, DOD has only recently begun to investigate PFAS contamination on and near its facilities.21 In the past several years, DOD has identified over 687 active or closed installations with known or suspected PFAS contamination.22 The Government Accountability Office (GAO) estimates remediation costs for these installations to reach more than $2.1 billion in 2021, in addition to $1.1 billion actual PFAS costs incurred in 2020. GAO projects that costs will increase significantly year over year.23 The House of Representatives version of the proposed 2021 National Defense Authorization Act (NDAA) allocates $549 million for PFAS cleanup at DOD installations.24
In addition, in 2020, DOD provided $40 million to the Agency for Toxic Substances and Disease Registry (ATSDR) to conduct exposure assessments of eight current and former military installations and a nationwide health study.25 The 2021 NDAA included an additional $15 million to support CDC and ASTDR's nationwide PFAS health study.26
C. PFAS Due Diligence
Thoroughly understanding the historical uses of a potentially contaminated site, along with the historical uses of PFAS in the surrounding area, is critical to identifying potential PFAS contamination associated with property development and transactions.27 "Phase 1" (document review) site investigations may miss the potential for PFAS contamination, as these chemicals were not historically considered hazardous.28 Once "Phase 2" soil and groundwater sampling at a site begins, it may remain difficult to identify the source of PFAS at a site, given the thousands of PFAS types and their different changes over time, and varying fate and transport mechanisms.29 In addition, many PFAS releases occurred decades ago, giving PFAS plumes time to develop.30 Further, many materials typically used for environmental sampling contain PFAS and, because many types of PFAS may pose risks even when present in small amounts, accurately sampling may be difficult.31 Regardless of these challenges, PFAS has already been incorporated into due diligence for property transactions, and the practice will necessarily
[Page 15-5]
expand given the growing concern and increased regulation surrounding these chemicals.
D. Developing Remediation Technologies
Thus far, PFAS water supply remediation projects have typically used carbon filters that more effectively catch long-chained PFAS; however, the filters are proving less effective for the short-chained substitutes.32 Even after PFAS have been removed from water or soil, PFAS-laden filters must be recycled or disposed, and unrecycled filters and other wastes must be disposed. Currently, much of this waste ends up in landfills, which can create additional contamination pathways as PFAS from waste disposal can seep into the ground, particularly in unlined landfills.33 Further research is needed to develop cost-effective destructive technologies for PFAS that result in complete...
To continue reading
Request your trial
